Low-temperature mineralization of perfluorocarboxylic acids
Eureka!
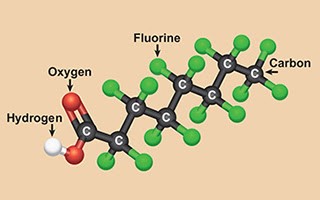
Researchers from Northwestern University (IL, USA) found that there is a potential weak spot in carboxylic acid–containing PFAS: Decarboxylation in polar, non-protic solvents yields a carbanion that rapidly decomposes. The authors used computational work and experiments to show that this process involves fluoride elimination, hydroxide addition, and carbon–carbon bond scission. The initial decarboxylation step is rate limiting, and subsequent defluorination and chain shortening steps occur through a series of low barrier steps.
…but in the real world.
It became world news. Everyone yearns for a way to clean up PFAS once and for all. But it’s not a quick fix, for the treatment of contaminated water or contaminated soil, the method is unsuitable, chemists and water purifiers say. The agent used to dissolve the PFAS in (dimethyl sulphoxide – abbr. DMSO) is a contaminant itself. Recovering DMSO from water is complex and takes a lot of energy. Also the high temperature needed forms a stumbling block. In their publication, Dichtel and colleagues talk about "mild temperatures" of 80 to 120°C but for a sewage treatment plant, 120°C is not mild. Heating the water would simply ask too much energy.
Not a ready-to-use solution
Moreover, PFAS are just one of the micropollutants that sewage treatment plants struggle with. Drug residues, microplastics and pesticides are present in low concentrations, just like PFAS. A combined approach using, for example, filters to remove all kinds of compounds from the water makes more sense than a separate treatment for PFAS. William Dichtel, researcher at Northwestern University who conducted the study, agrees: "This research is not going to solve the pollution problem. People are hoping for a breakthrough, but this is not a breakthrough. It is a step forward, an entry point. An exploration of new ways to destroy PFAS.”
The article “Low-temperature mineralization of perfluorocarboxylic acids” by Trang et al was published in SCIENCE, Volume 377 on August 19, 2022.
EmConSoil coordinator
- Address
- Stationsstraat 110
2800 Mechelen
Route and accessibility - Telephone
- +32 15 284 284
